Sample request
You would like to convince yourself? Request a free sample.
FAQ
General
What are FastGene UF Concentrators used for?
They are used for concentration, desalting, and buffer exchange of biomolecules such as proteins, nucleic acids, and viruses. They can also be used to clarify samples or remove small contaminants
What sizes and MWCOs are available?
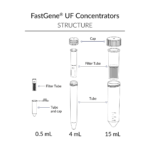
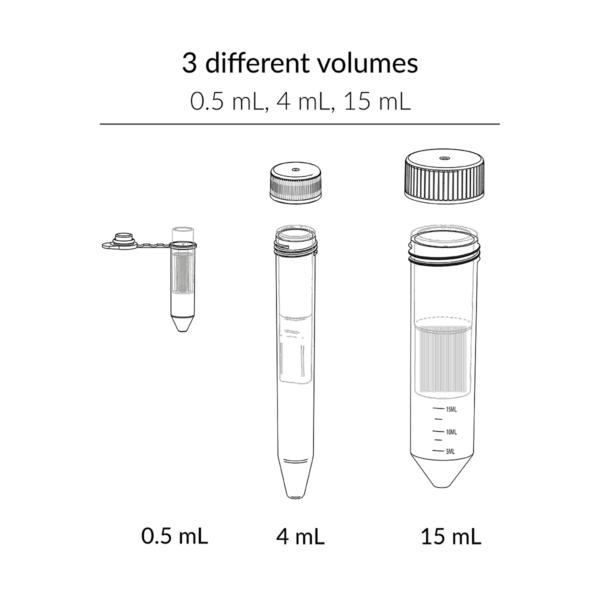
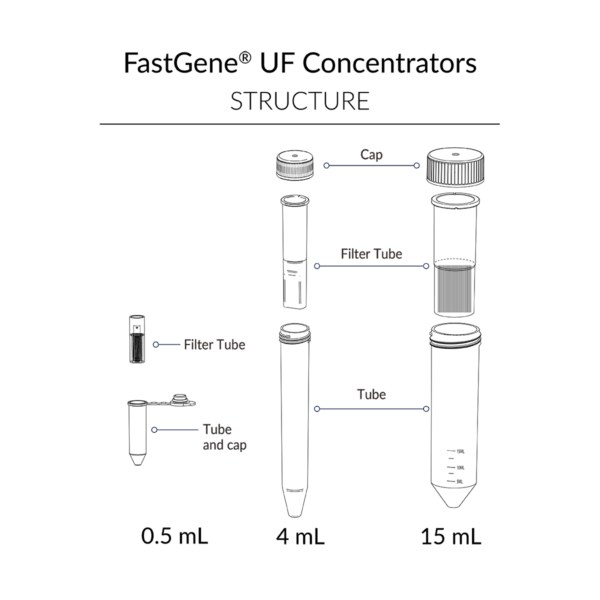
FastGene® UF Concentrators are offered in three sizes – 0.5 mL, 4 mL, and 15 mL – each in three molecular weight cut-offs (MWCO): 10 kDa, 30 kDa, and 100 kDa.
What membrane material is used?
All FastGene® UF Concentrators use a low-binding PES (Polyethersulfone) membrane, ideal for proteins and nucleic acids.
What pH range can they handle?
The PES membrane is compatible with pH 1–14, covering most biological and chemical applications.
Can I reuse the concentrators?
All FastGene® UF Concentrators are single-use, non-sterile devices. Reuse may reduce performance and risk contamination. Reuse is not recommended.
Can they be sterilized?
Do not autoclave. For cleaning or limited sterilization, you may rinse with 70 % ethanol for 30 minutes before use. Always let ethanol evaporate completely.
Are the concentrators RNase- or endotoxin-free?
They are not certified RNase-free or endotoxin-free. If required, rinse with 0.1 % DEPC solution at 37 °C for 2 h and then wash thoroughly with ultrapure water.
Are the devices chemically compatible with organic solvents or strong reagents?
They are intended for aqueous and biological solutions.
Check the chemical compatibility table (see user manual or list below) before using acids, bases, or solvents.
| Category |
Chemical / Reagent |
Max. concentration |
|
Category |
Chemical / Reagent |
Max. concentration |
| Acid |
Sulfamic acid |
≤3 % |
|
Organic solvent |
Benzene |
Not recommended |
|
Formic acid |
≤5 % |
|
|
Acetone |
Not recommended |
|
Acetic acid |
≤25 % |
|
|
Acetonitrile |
≤10 % |
|
Hydrochloric acid |
≤1 M |
|
|
Toluene |
Not recommended |
|
Sulfuric acid |
≤3 % |
|
|
Formaldehyde |
≤5 % |
|
Nitric acid |
≤10 % |
|
|
DMSO |
≤5 % |
|
Lactic acid |
≤5 % |
|
|
Ethyl acetate |
Not recommended |
|
Phosphoric acid |
≤30 % |
|
|
Pyridine |
Not recommended |
|
Trifluoroacetic acid |
≤10 % |
|
|
Chloroform |
Not recommended |
|
Trichloroacetic acid |
≤10 % |
|
|
Carbon tetrachloride |
Not recommended |
| Base |
Sodium hydroxide
(4 mL / 15 mL tubes) |
≤0.5 M |
|
|
Tetrahydrofuran |
Not recommended |
|
Sodium hydroxide
(0.5 mL tubes) |
≤0.1 M |
|
Other reagent |
Phenol |
< 1 % |
|
Ammonium hydroxide |
≤10 % |
|
|
Glycerol |
≤70 % |
| Alcohol |
Methanol |
≤60 % |
|
|
DTT |
≤0.1 M |
| |
Ethanol |
≤70 % |
|
|
DEPC (Diethyl pyrocarbonate) |
≤0.2 % |
| |
Isopropanol |
≤70 % |
|
|
PEG (Polyethylene glycol) |
≤10 % |
| |
n-Butanol |
≤70 % |
|
|
Phosphate buffer (pH 8.2) |
≤1 M |
| |
|
|
|
|
Ammonium sulfate |
Saturated |
| |
|
|
|
|
Imidazole |
≤500 mM |
| |
|
|
|
|
Urea |
≤8 M |
| |
|
|
|
|
β-Mercaptoethanol |
≤0.01 M |
| |
|
|
|
|
Tris buffer (pH 8.2) |
≤1 M |
| |
|
|
|
|
Sodium carbonate |
≤20 % |
| |
|
|
|
|
Guanidine hydrochloride |
≤6 M |
Usage
Can I use them at 4 °C?
Yes. Centrifugation at 4 °C is possible, but viscosity increases at low temperature, so extend the spin time by about 1.5 × times the original duration.
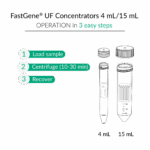
How do I collect the concentrated sample?
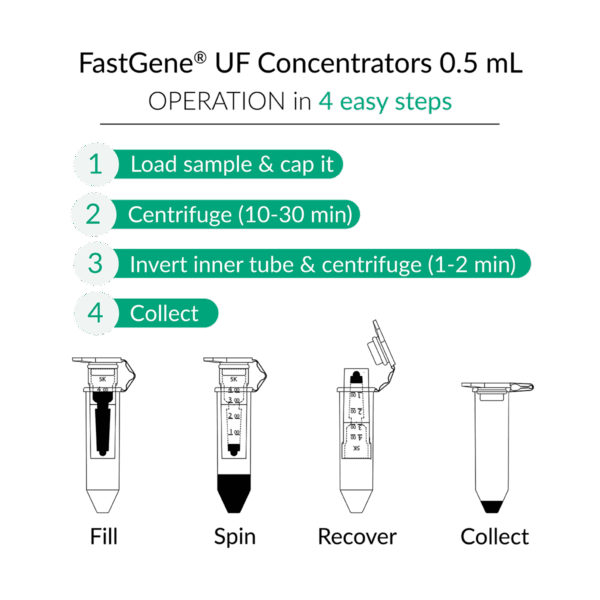
- 0.5 mL tubes: Use the reverse-spin method – invert the filter into a clean collection tube and centrifuge at 1,000 × g for 1–2 min.
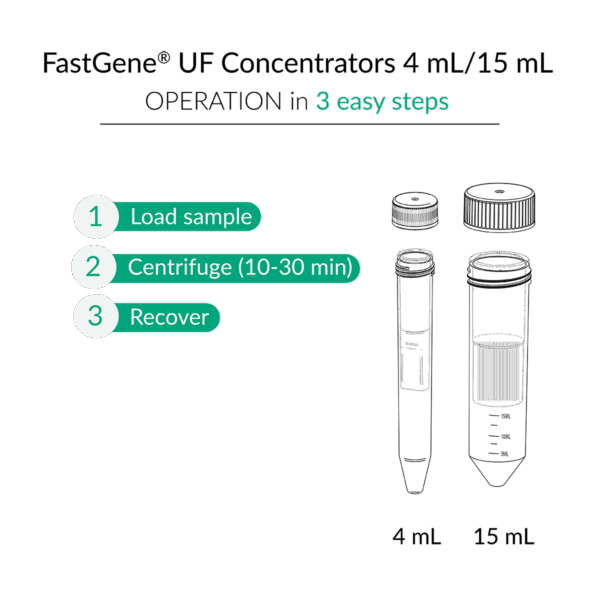
- 4 mL and 15 mL tubes: Collect the concentrate directly with a pipette.
How can I improve protein recovery or avoid loss?
- Choose an MWCO about one-third of the target protein’s molecular weight.
- Avoid excessive centrifugal force or long spin times.
- If the sample is viscous or prone to sticking, pre-block the membrane with 10 % glycerol overnight and rinse before use.
Can these devices remove detergents or endotoxins?
- Detergents: Only partially; efficiency drops above the CMC value.
- Endotoxins: No, as endotoxins are often >10 kDa and not removed effectively by ultrafiltration.
Can FastGene UF Concentrators be used for virus or nanoparticle concentration?
Yes.
- Lentivirus: Use 100 kDa MWCO.
- Adenovirus: Use 50 kDa MWCO.
They are also suitable for nanoparticle and exosome concentration (recommended 50–100 kDa).
What centrifuge settings should I use?
- 0.5 mL: Up to 14,000 × g
- 4 mL: Up to 6,000 × g (3,000 × g for 100 kDa)
- 15 mL: Up to 5,000 × g (3,000 × g for 100 kDa)
Use swing-bucket or fixed-angle rotors compatible with standard conical tubes. Always balance your samples.
Do samples dry out during centrifugation?
No. The FastGene® UF Concentrators feature a dead-volume design that prevents complete drying and protects your sample.
Troubleshooting
My protein precipitated during concentration — what should I do?
Precipitation can occur when concentrating too quickly or too far. Try:
- Reducing centrifugal force (to 30–50 % of the recommendation).
- Switching to a larger MWCO (e.g., 30 kDa instead of 10 kDa).
- Gently mixing the sample between spins.
Why is my target protein missing after centrifugation?
Possible causes:
- The MWCO chosen is too large (target passed through).
- Centrifugal force too high or rotor not calibrated.
- Protein precipitated during spin. Check filtrate and concentrate; if protein is in filtrate, use a smaller MWCO.
Only logged in customers may leave a review.






Reviews
There are no reviews yet.