Details
MIDORI Green Advance
MIDORI Green Advance is a safe alternative to the traditional nucleic acid stain ethidium bromide. It is a non-carcinogenic and less mutagenic dye for detecting dsDNA, ssDNA and RNA in agarose gels with a very high sensitivity. MIDORI Green Advance can utilize with UV light or with our innovative Blue/Green LED technology.
Safe alternative to ethidium bromide
MIDORI Green Advance is a non-carcinogenic dye. Optimised for a brighter signal when excited by UV-light or Blue/Green light. It has the advantages, such as being non-carcinogenic and
having an excellent signal-to-noise ratio.
MIDORI Green Advance shows a very high sensitivity even for small DNA fragments. The dilution factor of MIDORI Green Advance can be as high as 1:25000. Hence, 4-6 μL are enough for the staining of a 100 mL agarose gel, resulting in ~17 to 25 liters of stained agarose gels.
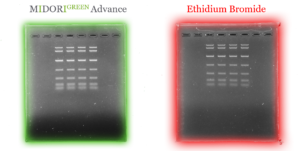
Fig. 1: Comparison of sensitivity between MIDORI Green Advance (left green) and ethidium Bromide (right red) using a UV-transilluminator.
Proven safety
It is essential for a good replacement of the mutagenic DNA stain ethidium Bromide to deliver strong signals. MIDORI Green Advance delivers signals with a comparable intensity. Nonetheless, the safety of the user must not be compromised. Hence, several tests were performed with MIDORI Green Advance and according to those tests, MIDORIGreen Advance is safe.
- Ames-Test
- Acute Oral Toxicity Test
- Mouse Bone Marrow Micronucleus Test
- Chromosome Aberration Test
- Latex and Nitrile Gloves Penetration
Download here the MIDORI Green Advance safety report.
Data
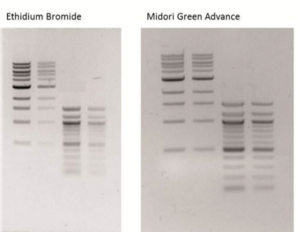
Comparison of signal intensity of Midori Green Advance and Ethidium bromide visualized by UV-Light.
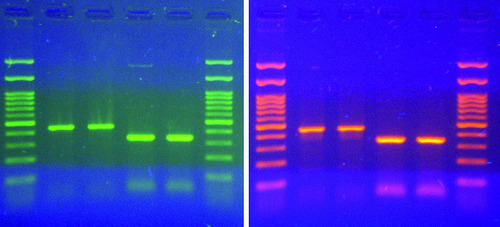
Fig. 1: Midori Green Advance- (left) and Ethidium Bromide-signal intensity (right) on a UV-Light Illuminator
Comparison of signal intensity of Midori Green Advance and other stains visualized by Blue/Green LED light and UV-Light
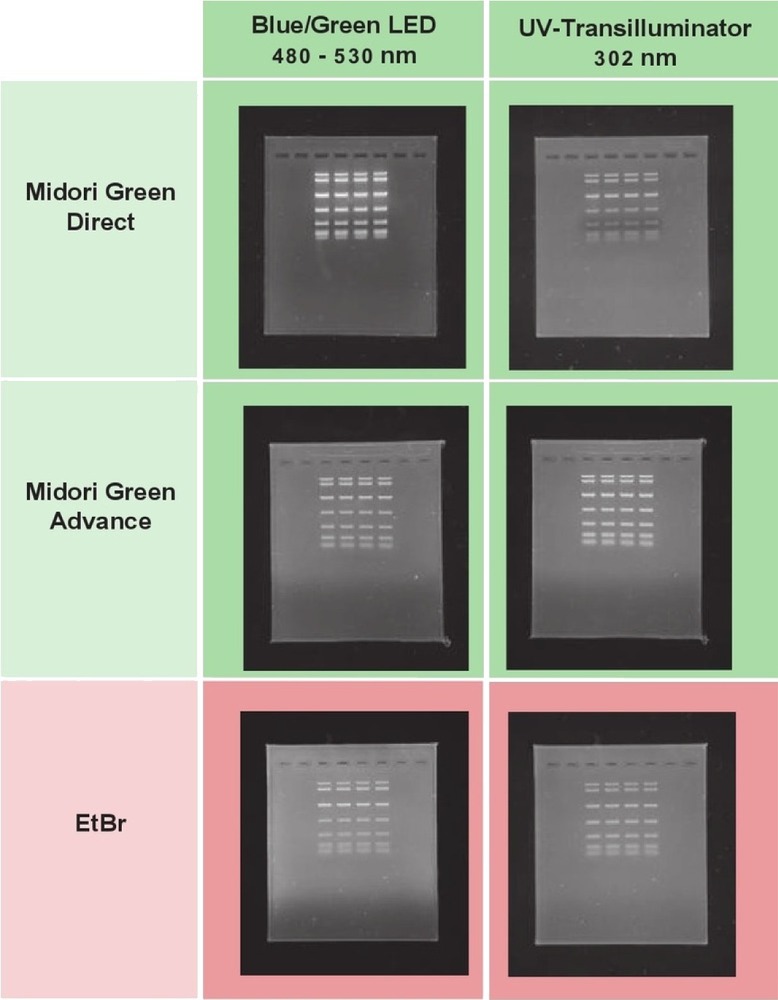
Fig. 2: Signal intensity of Midori Green Advance; Midori Green Direct and Ethidium Bromide on a Blau/Grün LED Transilluminator compared to a UV-Light Transilluminator
Customer Feedback
More than 500 citations in journals!
MIDORI Green Advance has been used very successfully in many laboratories. The feedback from the scientific society was very positiv. Enclosed a few results from independent scientists:
Customer #1
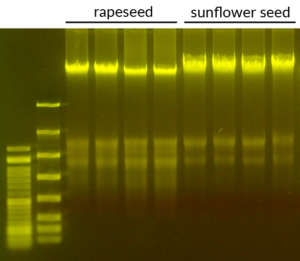
gDNA was extracted from rapeseed and sunflower seed using the Clean Plant PK Kit.
Customer #2
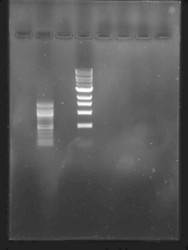
“…we have tested Midori Green Advance and we are very excited about it. Please find attached to this email a typical picture….”
Data kindly provided by: Dr. Olaf Voolstra, Biosensor Research Group, Hohenheim University Stuttgart, Germany
Customer #3

Ethidium bromide (left) in comparison with MIDORIGreen Advance (right) on UV table
Data kindly provided by: Mr. Schuster, Friedrich-Alexander-University Erlangen-Nürnberg, Germany
Customer #4
Comparison of ethidium bromide and MIDORI Green Advance on a UV table
Data kindly provided by: Ms. Abel, Heinrich-Heine-University Düsseldorf, Germany
Protocols
In-gel staining
- Prepare 100 mL of agarose gel solution (concentration from 0.8-3.0%) and heat until the solution is completely clear and no small floating particles are visible.
- Add 4-6 µL of MIDORI Green Advance DNA Stain to the gel solution and mix it gently. Cool the gel to 50- 60ºC and cast the gel, into the gel tray. When the gel is solid, load the samples and perform electrophoresis.
- Detect the bands using a UV or LED illuminator. Yellow or green gelatin- or cellophane filters should be used for photography.
Poststaining
- Data kindly provided by:Advance DNA Stain poststaining solution may be used 2-3 times.
- Staining solution that is going to be reused should be preferably stored at room temperature in the dark.
- For <0.5 cm thick agarose gel, 10-25 µL of the stain should be used per 100 ml of buffer.
- Optimal staining time (5-60 minutes) and the amount of the stain may depend on the thickness of the gel.
NOTICE: Usage is not recommended with SDS containing loading buffers because of band appearance caused by stain and SDS interaction!
FAQ
Can I use Midori Green Advance with Pulse Field Gel Electrophoresis (PFGE)? Normaly I used a poststaining with EtBr.
A poststaining is done with MGA by protocol which you can find in the protocol section.
Do you have any details about the disposal of Midori Green Advance please? I understand that the molecule is light-sensitive and I wondered if the MGA-gel could be exposed to light after use before disposal?
MGA is not toxic and it is sufficient to dispose it as chemical lab waste. The emissivity of MGA will be destroyed by light, but that does not mean that the chemical structure will be changed. But it is not necessary to destroy the molecule because it is anyway not harmful. That is the big advantage in comparison to ethidium bromide – that there is no special disposal necessary.
Why can’t MGA and MGD be used the same way?
The chemical structures are completely different. we tested to use MGD as MGA and vice versa. We can’t recommend both applications. As you try to use MGD as MGA, you need a lot of MGD and ladder in order to get observable bands. Samples with a low concentration could be stay undetected by this way. MGD was designed for addition to sample and is much less concentrated than MGA. It is very uneconomic to use MGD in the way like MGA – you need more than the 10 fold amount in the gel in order to observe bands. As you try to use MGA as MGD it is also problematic, because MGA is too high concentrated and is running in the opposite direction. I tried to reduce the MGA concentration, in this case you see some bands, but they are very weak independent which dilution is used. Summarizing you can say both stains are designed (with different structural formula) and optimized for different applications (in gel and addition to a sample) and it is not recommended to try to use them in another way.
What is the difference between MGA and MGD and how stable are these stains?
The main difference is that Midori Green Advance (MGA) is for in gel and post staining (means it is used like ethidium bromide) and Midori Green Direct (MGD) is added directly to the sample. Depending on the gel documentation system we recommend to use one or the other but you can use both for every illuminator (UV 302, 365 nm, Blue illuminator and Blue/Green transilluminator) – only the performance can be increased to an optimal level. For example we recommend to use MGA if the user has a UV transilluminator, because the results are fantastic with that stain. A really great performance you get with our Blue/Green LED instruments and MGD – no background at all and an intensive signal. Both stains are stable on room temperature, so shipping at RT is no problem, but we recommend to store the stains at 4°C.
Are MGA and MGD working with Agarose and acrylamide gels?
Our customers gave us the feedback that MDG and MGA (post staining) are working for acrylamide gels but primarily they are designed for agarose gels.
What happens if I stored MGA at -20°C for 6 days? Will it be OK for use?
The freezing destroys the functionality of MGA. You can still try but our experience it forfeits the illumination after freezing.
I use Midori Green Advance in Agarose Gels, why are the small bands invisible when I run a gel for 35 minutes and what do you recommend?
Midori Green Advance is possitively charged and runs in the different direction than the DNA, therfore the gel gets destained from the bottom. To see also small bands you can reduce the running time to 25 minutes. If you want to run your gel for a longer time, than you can post stain. You can use the in gel staining and then have a shorter time for post staining or you only post stain your gel without a previous in gel staining. In both cases the whole gel will be stained and also very low molecular weight bands are observable. You can also use Midori Green Direct instead of Midori Green Advance. This stain is added to the DNA so every band is stained no matter how long you run the gel. Another advantage is the nonexistent background. In special if you use blue or Blue/Green LED light instead of UV, you will get amazing signals.
On the Midori Green Advance Datasheet it is described that postaining solution may be used up to 2-3 times. For how long could you use the postaining solution without losing too much sensibility?
Actually, we have customers who use it more often than 2-3 times. If the staining bath is darkened the whole time, you can use it in any case 2-3 times without any reduction of signal intensity. But it is also possible to use it 5 – 8 times without a signal decrease depending on the size of the gel and the amount of samples. If you detect a decrease of intensity, it is possible to add a little bit MGA and thus get same signal intensity as before.
Can I use MGA after expiry date?
Yes, you can use it. The expiry date is just the minimal amount of time we guarantee but normally it last much longer.
I get some additional bands after using MGA?
This is possible, if you use SDS in the loading buffer.
Can you use Midori Green in a Polyacrylamid/Urea Gel?
This works really nice.
Is Midori working in Urea/Formamide gels?
Unfortunately, it is not working. That’s because of the Formamide. Urea gels are working.
Does Midori Green contain DMSO?
Our Midoris are DMSO free.
Can I use marker containing Orange G, Bromphenol blue or Xylen cyanol FF with Midori Green Advance?
Yes, you can use them. They do not cause probles, only Bromphenol blue can reduce the signal intensity or can lead to formation of clouds in the gel.
Can I add Midori Green Advance to the running buffer to reduce the destaining of the gel? If yes in which amount should I use it?
You can use Midori Green Advance in the running buffer. Here you should use arond 8 µL per 100 ml of buffer.
Can I use Midori Green Advance with further downstream applications like southern blots?
Customers tell us that there the dye does not interfere with standard Southern-protocols. We have no testing at our side, since there is no indication that the dye intherferes with procedures downstream of agarose gel-runs and – documentation.The only (indirectly) related issue is certainly, that no direct comparison can be drawn between Southern-blots that have been stained in-gel (during the electrophoresis) with EtBr in first experiments and later experiments that employed MG advance, since gel-run patterns are not directly comparable between these two dyes (if used in-gel). For any post-run staining and down-stream application there are no known issues.
Can Midori Green Advance be used in denaturating Formaldehyde Gels for separating RNA?
Yes, it can be used:
We have separated total RNA from 1% Formaldehyde gel with TAE buffer using both ways Post staining and in Gel Staining. In each case we used 5 microliters per 100 ml buffer. Post staining was carried out at room temperature for 1 hour.
The minimum RNA concentration should be at least 250 ng per line. The good working RNA concentration in 500 ng.
Only logged in customers may leave a review.















bob.kauffmann –
I (student) feel comfortable working with non-carcinogenic DNA staining products. The bands are also clearly visible.
Verified User
Rubén Arroyo, CINVESTAV –
Great signal for DNA, but rather weak for RNA (using formaldehyde-containing loading buffer), still not as good as EtBr.
Comment from Nippon Genetics:
Dear Mr Arroyo,
Many thanks for your feedback. I am glad to see that you were able to obtain very good results in staining DNA with MIDORI Green Advance. Regarding the results with RNA, I would like to suggest to you a publication from the year 2014:
Influence of trypsinization and alternative procedures for cell preparation before RNA extraction on RNA integrity.
The paper describes the use of MIDORI Green Advance for the staining of RNA separated using bleach RNA gels. Fig. 1-7 show agarose gels that were stained with MIDORI Green Advance.
An explanation for the difference that you experienced when comparing the signal of EtBr to MIDORI Green Advance could be due to the transilluminator used. We would like to invite you to see the signal of a gel you have prepared using our Blue/Green LED transilluminator or our FastGene UV-light table.
Best regards
Dr. Marcelo Lanz
Head of Product Management
Verified User
Physiologisches Institut, LMU –
Wir sind sehr zufrieden mit Midori Green Advance, perfekte alternative zur EtBr.
Translation by Nippon Genetics:
We are very happy with Midori Green Advance, which is a perfect alternative to EtBr.
Verified User
Dr Sandra Hoffmann, Institut für Humangenetik, Universität Heidelberg –
Midori Green Advance liefert sehr robuste Ergebnisse, welche absolut mit EtBr vergleichbar sind. Wir haben EtBr aus unseren Laboren mittlerweile vollständig durch Midori Green Advance, sowie Midori Green Direct ersetzt.
Translation by Nippon Genetics:
Midori Green Advance delivers very good results that are absolutely comparable to EtBr. We replace completely EtBr from our laboratories with Midori Green Advance and Midori Green Direct.
Verified User
Dr. Sturm, Applied Biosciences, KIT –
Absolutely the best alternative to EtBr. We changed many years ago from EtBr to Midori and since then, we never had any issues. Signals are as good as before and low toxicity works well with student environment.
Verified User
D. Tacke, Georg-August-University Göttingen, plant pathology and plant protection –
Wir verwenden Midori Green Advance seit 2014 in unserer Abteilung und sind sehr zufrieden damit. Sowohl im täglichen Laboralltag als auch in Studentenpraktika liefert der Farbstoff sehr gute Ergebnisse.
Translated by Nippon Genetics:
We use Midori Green Advance in our department since 2014 and are very satisfied with it. In everyday laboratory practice as well as in practical course for students the stain delivers very good results.
Verified User
Dr. Olaf Voolstra von der Universität Hohenheim, Fg. Biosensorik aus Stuttgart –
„…haben wir das Midori Green Advance getestet und sind begeistert!… ”
Translated by Nippon Genetics:
“…we have tested Midori Green Advance and we are very excited about it….”
Verified User