Details
MIDORI Green Direct is a safe alternative to ethidium bromide for visualization of double-stranded DNA, single-stranded DNA, and RNA in agarose gels. In contrast to most other non-ethidium bromide based dyes, MIDORIGreen Direct is just added to your samples. The loading dye is already included. You do not need to add any other loading dye to both gel matrix and running buffers
- Direct Staining of DNA/RNA
- No toxicity, non-carcinogenic
- Safe alternative of Ethidium Bromide
- Loading Dye is included
- Very low Background
Get the best signal to noise ratio
The direct staining of the DNA rather than the gel eliminates the background staining providing a perfect signal (Fig. 1). MIDORIGreen Direct was developed to work with Blue Light LED illuminators, but you can also use it with a regular UV transilluminator.

Fig.1: MIDORI Green Direct detected by blue/green LED light vs ethidium bromide detected using UV-light.
Better Results for Downstream Application
The isolation of DNA from agarose gels enables downstream applications. It is well known that many dyes, such as ethidium bromide or even SYBR™ Green are strong enzyme inhibitors due to their intercalating properties. MIDORI Green dyes bind to the DNA backbone. This results in a much higher efficiency for downstream applications, i.e. cloning (Fig. 2), seqeuncing, PCR, etc.
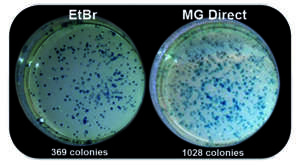
Fig.2: Better cloning efficiency with MIDORI Green Direct. Increased number of transformed E. coli colonies with MIDORI Green Direct than with ethidium bromide.
Download here our application note for more information
Proven Safety
MIDORI Green Direct stain is non-carcinogenic and less mutagenic compared to ethidium bromide. Furthermore, we can state that MIDORI Green Direct is impenetrable to latex gloves and cell membranes.
MIDORI Green Direct is classified as non-hazardous to aquatic life, under CCR Title 22 regulation. Thus, small amounts of MIDORI Green Direct stain can be safely released into the environment.
- Ames-Test
- Cytotoxicity
- Cell membrane Permeability
- Hazardous Waste Screening
- Latex and Nitrile Gloves Penetration
Download here the MIDORI Green Direct safety report.
Loading Dye
MIDORI Green Direct stains are provided in a form of 10X sample loading dyes and they are to be added to your samples only. You do not need to add any other dyes to the gel matrix nor to the running buffers.
Applications
Staining products after a restriction digest
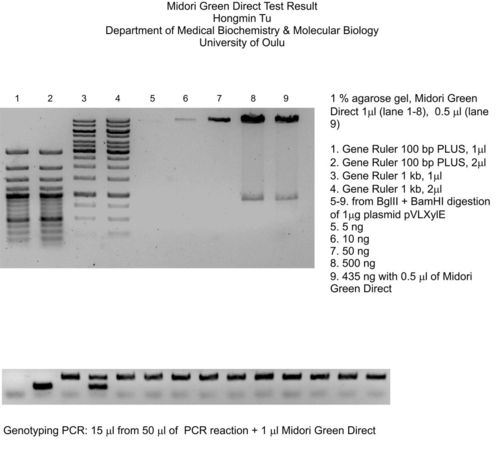
Staining differents DNA Ladder concentrations

Fig.2: 1% agarose gel – samples were directly stained with MIDORI GREEN DIRECT
FAQ
Why can’t MGA and MGD be used the same way?
The chemical structures are completely different. we tested to use MGD as MGA and vice versa. We can’t recommend both applications. As you try to use MGD as MGA, you need a lot of MGD and ladder in order to get observable bands. Samples with a low concentration could be stay undetected by this way. MGD was designed for addition to sample and is much less concentrated than MGA. It is very uneconomic to use MGD in the way like MGA – you need more than the 10 fold amount in the gel in order to observe bands. As you try to use MGA as MGD it is also problematic, because MGA is too high concentrated and is running in the opposite direction. I tried to reduce the MGA concentration, in this case you see some bands, but they are very weak independent which dilution is used. Summarising you can say both stains are designed (with different structural formula) and optimized for different applications (in gel and addition to a sample) and it is not recommended to try to use them in another way.
What is the difference between MGA and MGD and how stable are these stains?
The main difference is that Midori Green Advance (MGA) is for in gel and post staining (means it is used like ethidium bromide) and Midori Green Direct (MGD) is added directly to the sample. Depending on the gel documentation system we recommend to use one or the other but you can use both for every illuminator (UV 302, 365 nm, Blue illuminator and Blue/Green transilluminator) – only the performance can be increased to an optimal level. For example we recommend to use MGA if the user has a UV transilluminator, because the results are fantastic with that stain. A really great performance you get with our Blue/Green LED instruments and MGD – no background at all and an intensive signal. Both stains are stable on room temperature, so shipping at RT is no problem, but we recommend to sotre the stains at 4°C.
Are MGA and MGD working with Agarose and acrylamide gels?
Our customers gave us the feedback that MDG and MGA (post staining) are working for acrylamide gels but primarily they are designed for agarose gels.
Can you use Midori Green in a Polyacrylamid/Urea Gel?
This works really nice.
Is Midori working in Urea/Formamide gels?
Unfortunately, it is not working. That’s because of the Formamide. Urea gels are working.
I use Green Taq from Promega with the dye. Could the dye create some kind of conflict with the use of MGD?
It depends which illuminator you are using. There will be no problem with a UV-light illuminator because the dye won’t be visible. But if you use blue/green LED illumination it is possible that you can detect the dye form the Promega Green Taq. If it is a bad luck the band of the sample is as big as the dye band. In this case the dye band would overlay the DNA band. But generally there should be no interaction between Midori and the Promega dye. So as long as UV-light is used or the DNA band has not the same size as the dye band there should be no problem.
Can MGD bind to DNA of uncleaned PCR products?
Yes, it can.
Only logged in customers may leave a review.












Olivia Masseck, Fakultät für Biologie & Biotechnologie, Universität Bochum –
“Midori Green Direct ist sehr gut detektierbar und lässt sich auch wenn nötig mit UV Licht anregen. Gleichzeitig ist es überzeugend einfach in der Anwendung.”
Translation by Nippon Genetics:
“Midori Green Direct is very good detectable and can be excited with UV light if necessary. At the same time it is convincing by the easy handling.”
Verified User